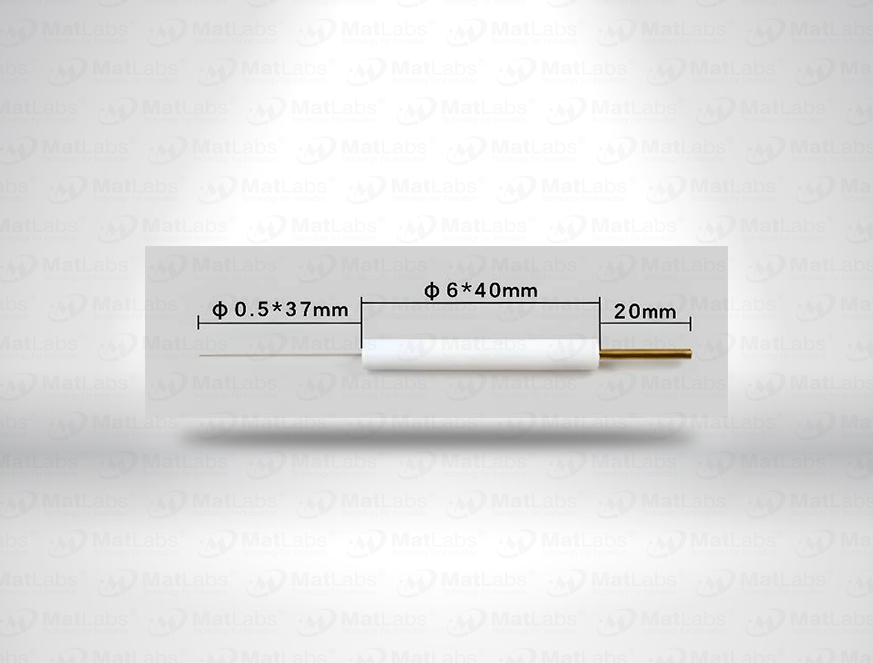
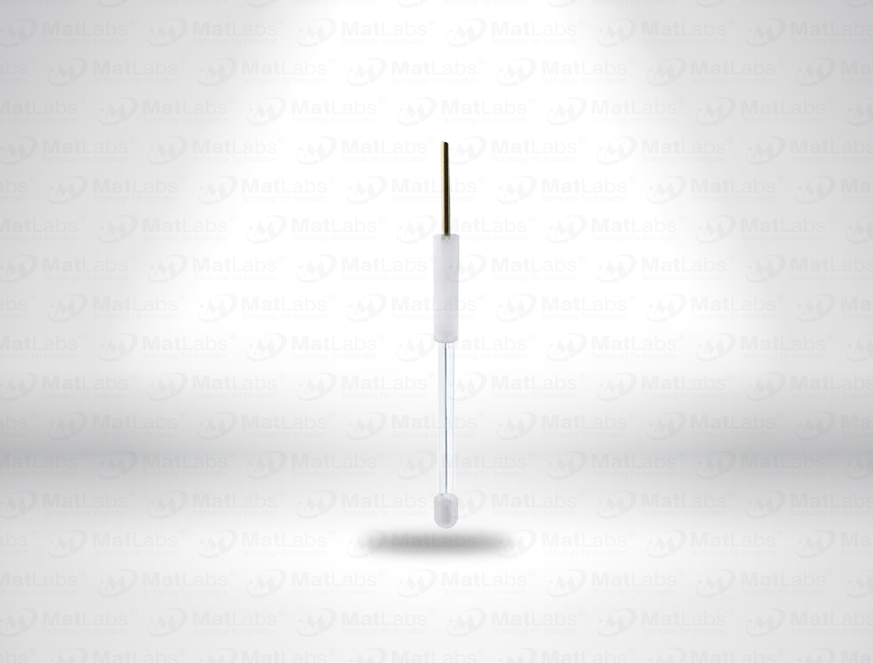
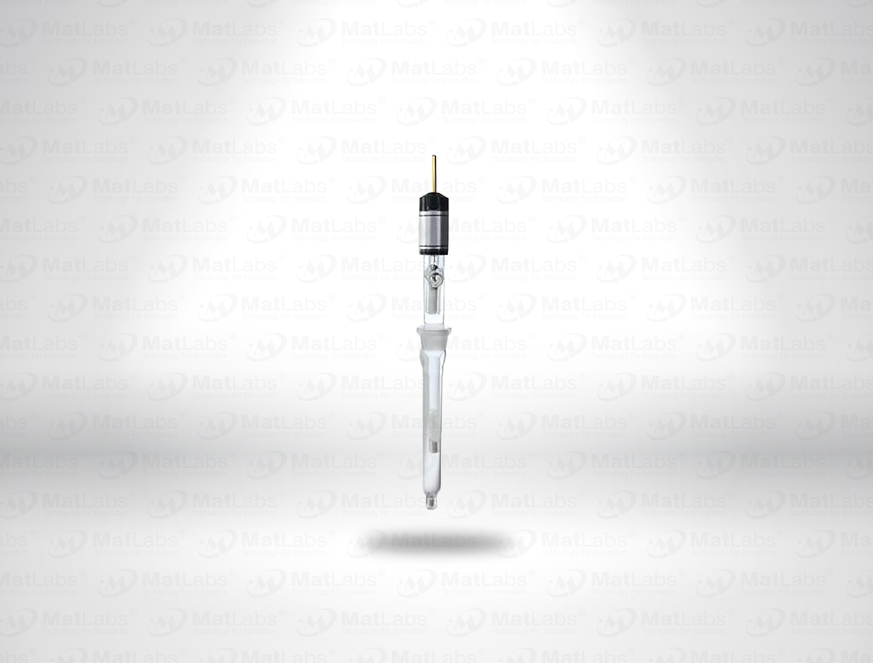
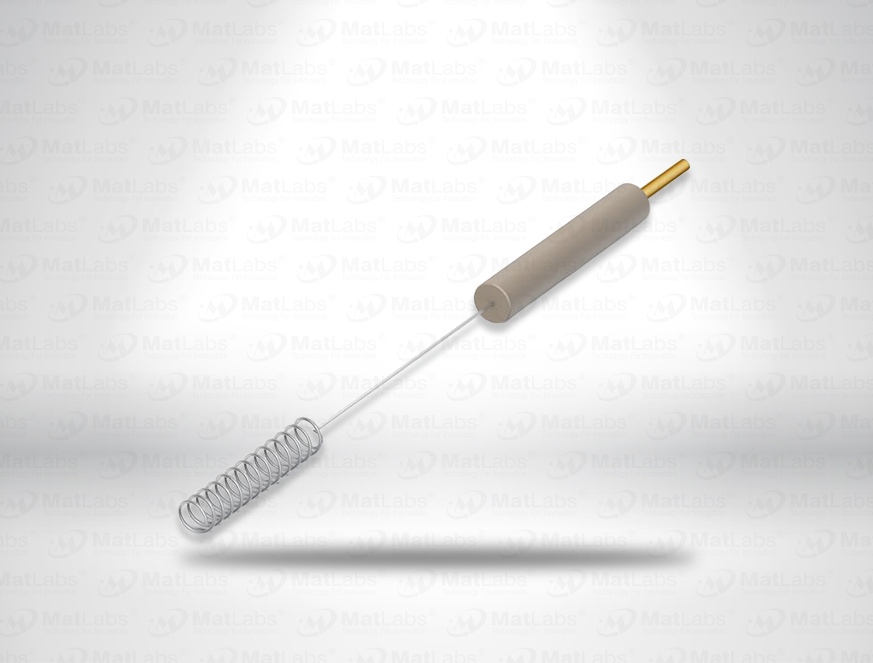
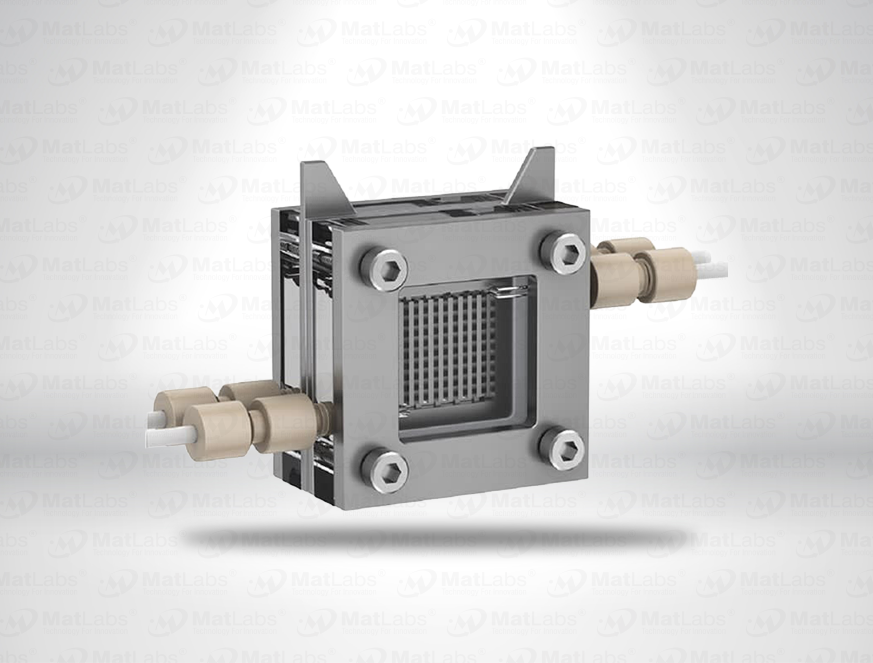
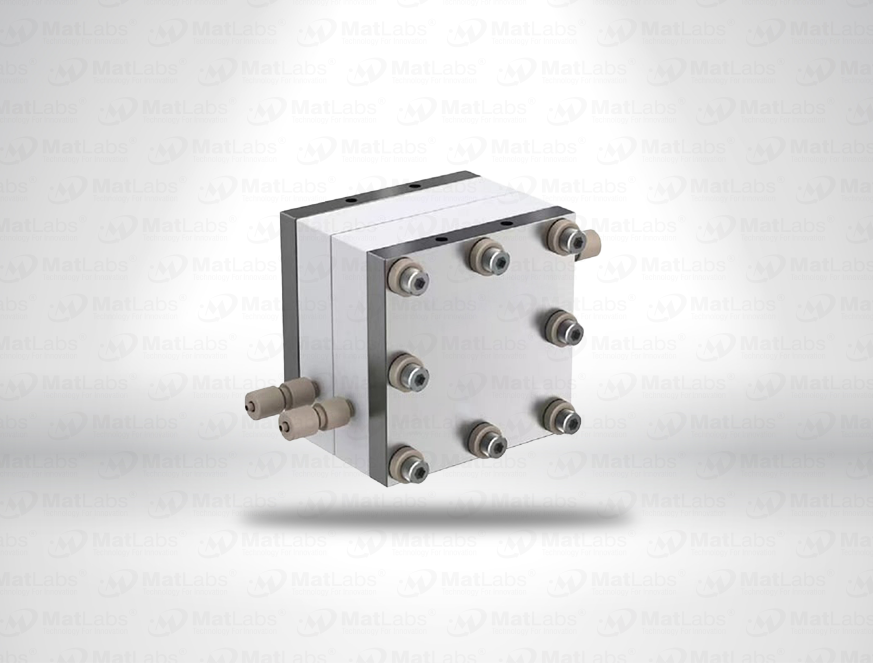
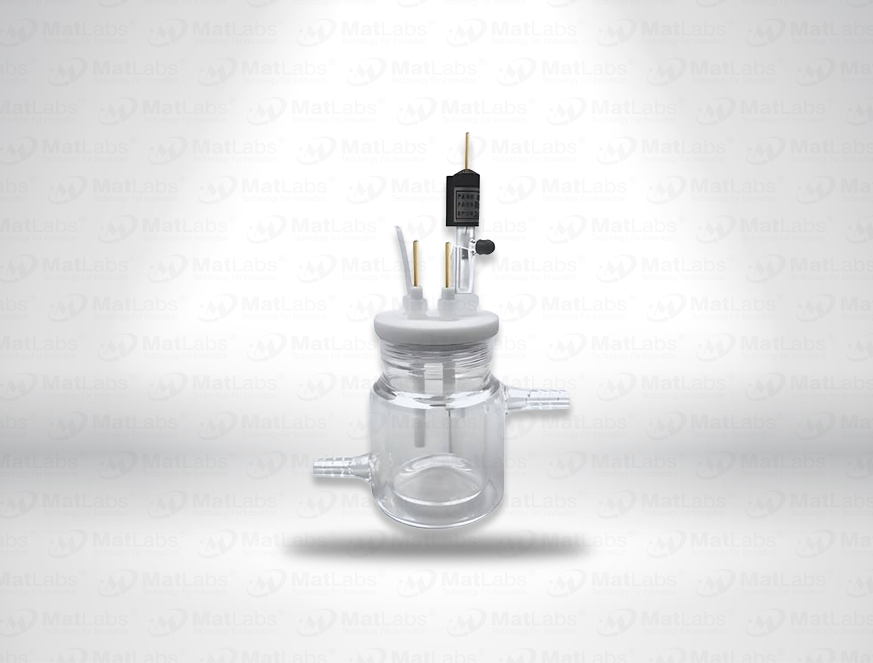
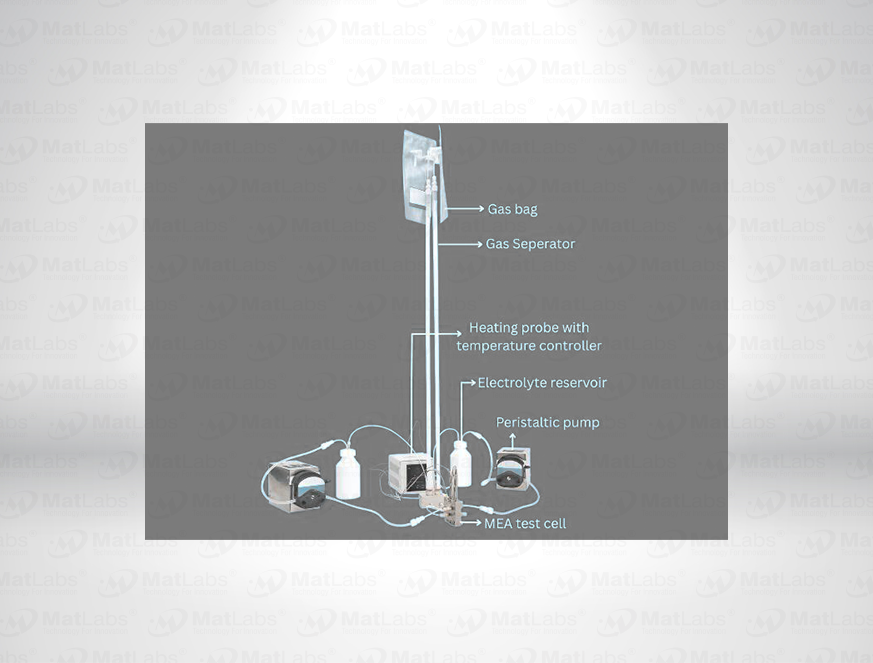
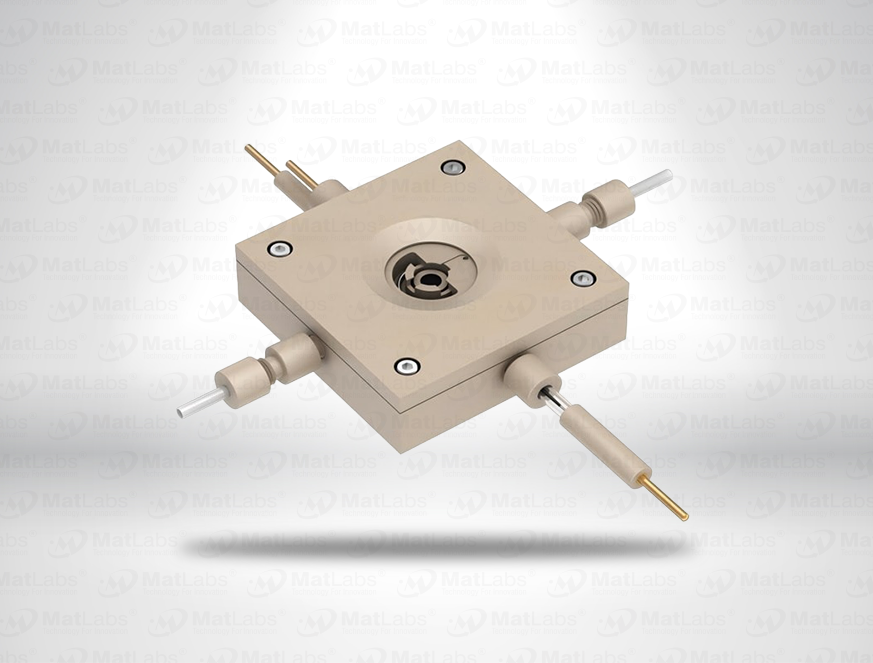
The reversible hydrogen electrode provides an in-situ hydrogen-coupled reference, aligning to the SHE scale with a pH-dependent potential (E(RHE) = E(SHE) + 0.059 pH at 25 °C). RHEs are widely used to benchmark electrocatalysts (HER/OER/ORR) because they track proton activity directly in your electrolyte. A typical design polarises a Pt element in an acid compartment while continuously generating H2; double-junction configurations enable operation in neutral media with minimal crossover. For best practice: ensure adequate H2 supply and stable pH/ionic strength; locate the reference close to the working electrode; and verify potential against a known standard if switching electrolytes. Use where reporting vs RHE is required by your community or journals.